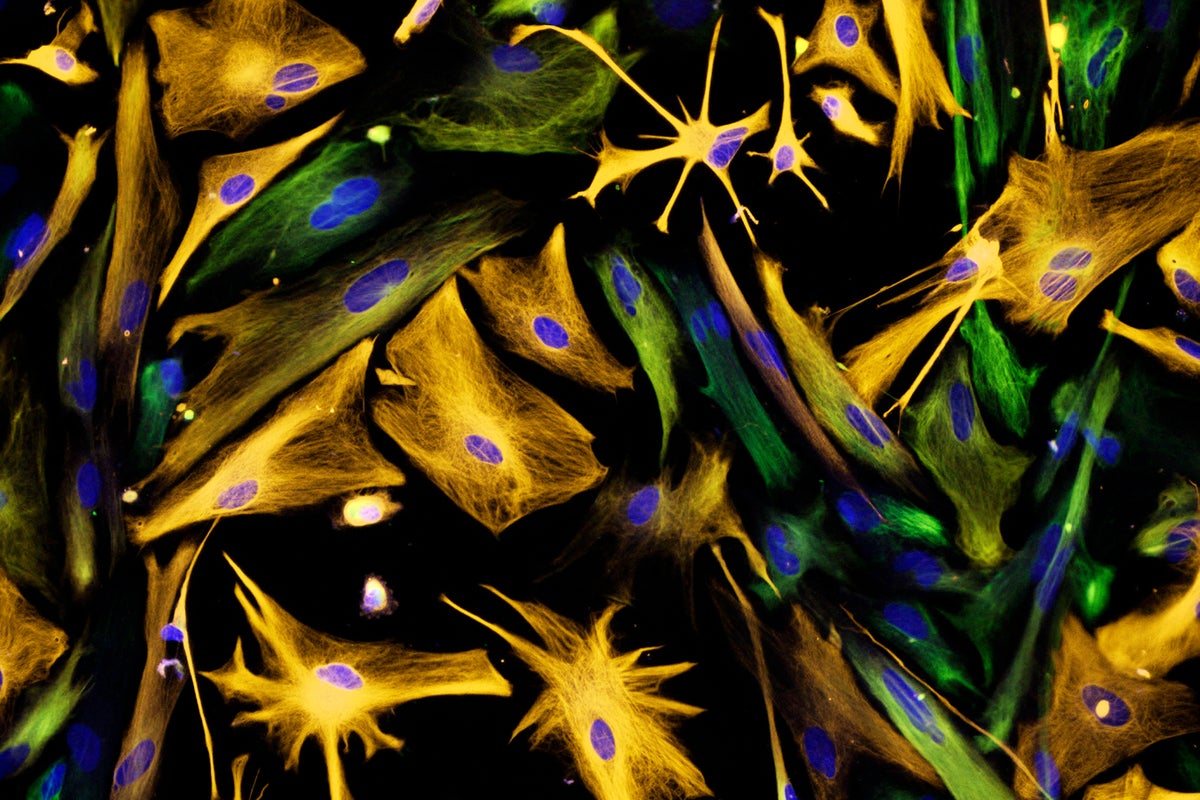
Proof That Adult Brains Make New Neurons Settles Scientific Controversy
Adult brains grow new neurons, and scientists have finally pinpointed where they come from
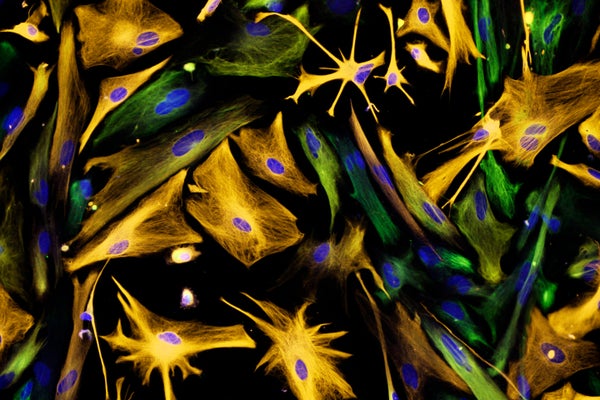
Neural precursor cells (green) are have been difficult to identify in human brians.
Carol N. Ibe and Eugene O. Major/National Institutes of Health/Science Source
For at least six decades, neuroscientists have been arguing over a big, foundational question: Do adult brains make new neurons? This process of “neurogenesis” had been shown in other adult animals, but its evidence in humans was circumstantial—until now. Using a new technique, scientists have found newly formed neurons in the brains of adults as old as age 78—and, for the first time, have identified the other brain cells that birthed them.
The results, published on Thursday in Science, are the first signs that cells with the capacity to turn into neurons, called neural precursor cells, exist in adult human brains. “Now we have very strong evidence that the whole process is there in humans, from the precursor cells to the immature neurons,” says Gerd Kempermann, a neurobiologist at the Dresden University of Technology, who was not involved in the study.
Throughout gestation, our brain churns out new neurons until it reaches the 100 billion we start life with, and that count declines as we age. As early as 1962, studies in rats had shown that neurogenesis continued throughout the animals’ life. Others found that young neurons existed in adult human brains. But it was unclear whether these “immature” neurons were truly new—or whether humans just start life with a collection of them, after which they slowly develop during adulthood.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
One thing was clear from these studies: if adult neurogenesis happened anywhere, it was in the hippocampus, a deep-brain structure known for its role in memory processing and storage. But even in the human hippocampus, neuroscientists had not yet found the precursor cells that divide and develop to turn into new neurons.
Researchers at the Karolinska Institute in Sweden had previously found immature neurons in the human brain. Marta Paterlini, a neuroscientist at the institute, and her colleagues, set out to pin down how those neurons came to be. Paterlini and her team took advantage of a new combination of techniques to examine immature neurons and neural precursor cells in the hippocampi of six young children, whose brain had been donated to science upon their death. From more than 100,000 cells, the researchers sequenced RNA—bits of genetic information used to carry out actions within each cell. These markers come together to form a sort of molecular fingerprint that can be used to predict a cell’s stage of life. “It’s not a matter of one marker defining active neurogenesis; it’s the combination of many markers,” says Paterlini, who is co-lead author of the new study.
After identifying these markers in young brains, the team then searched for those same signatures in 19 postmortem brains ranging from 13 to 78 years old. All of the brains contained immature neurons except one. The researchers also found neural precursor cells in each of the child brains and in 12 of the 19 adolescent and adult brains.
Two adults stood out for having many more neural precursor cells and immature neurons than the rest. The younger of these two people had lived with epilepsy, which could potentially connect to the apparent abundance of neurogenesis. In mice, higher levels of neurogenesis can cause seizures, though the connection to epilepsy in humans is still unclear.
The team suspects that neurogenesis happens in other parts of the adult brain, too. In mice, new neurons are regularly made in the olfactory bulb (a structure that processes smells) as well—but the same hasn’t been shown in humans. Paterlini plans to investigate whether adult neurogenesis might happen there or elsewhere in the brain.
Some research in mice suggests that disrupted neurogenesis is linked to Alzheimer’s disease and depression. Learning more about how neurogenesis happens—and whether the process can be altered—could prove helpful for understanding a range of disorders and diseases, says the study’s co-lead author Ionut Dumitru, a neuroscientist at the Karolinska Institute.
With the question of adult neurogenesis resolved, scientists can begin learning more about what neurogenesis does in the brain and how it affects various disorders. “This is an important paper because it should finally put this all to rest,” Kempermann says. “And we can now concentrate on the question: How do these cells in the human contribute to brain function?”